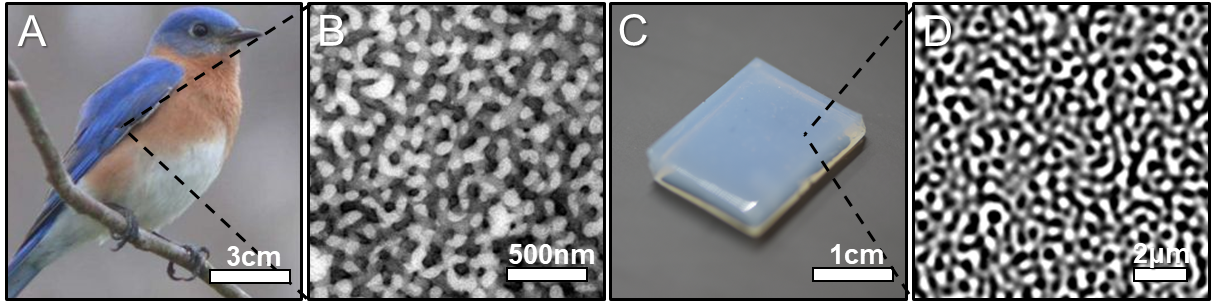
The eastern bluebird isn’t simply beautiful to look at. Its feathers also feature a unique structure that could revolutionise sustainable applications such as batteries and water filtration.
Specifically, the brilliant blue of the bird’s wings isn’t the result of colour pigmentation. Instead, it’s due to a network of channels with a diameter of a few hundred nanometres, traversing the feathers.
This network structure inspired researchers at ETH Zurich to replicate this material in the lab. They have now developed a synthetic material that exhibits the same structural design of the bluebird’s feathers — with the potential to deliver practical use cases, such as improved battery life.
The researchers experimented with a transparent silicone rubber that can be both stretched and deformed. They placed it in an oily solution, leaving it to swell for several days in an oven heated at 60 °C. They then cooled and extracted it.
The team observed that the rubber’s nanostructure had changed during the procedure and they identified similar network structures to the ones in the bluebird’s feathers. The only essential difference was the thickness of the formed channels: the synthetic material was 800 nanometres next to the feather’s 200 nanometres.
The achievement was a result of the new method based on the phase separation of a polymer matrix and an oily solution. Phase separation is a common physics phenomenon we’ve all witnessed in our everyday life. For instance, think of a salad dressing made of oil and vinegar — the substances separate unless vigorously shaken and separate again when the shaking stops.
But it’s also possible to mix the substances with heating and separate them again with cooling — and that’s exactly what the scientists did in the lab.
“We are able to control and select the conditions in such a way that channels are formed during phase separation. We have succeeded in halting the procedure before the two phases merge with each other completely again,” said Carla Fernández Rico, lead author of the study.
A notable advantage of this method is that the material remains scalable. “In principle you could use a piece of rubbery plastic of any size. However, you’d then also need correspondingly large containers and ovens,” added Rico.
The technology could prove useful in batteries by replacing liquid electrolytes, which facilitate the transfer of lithium ions between the electrodes, but often react with the ions and, this way, reduce battery capacity and health. Solid electrolytes with a network structure like the one developed by the researchers would not only eliminate the issue, but also enable good ion transport and increase battery life.
Water filters are another potential field of application thanks to the network’s transport properties and large surface area, which could enable the removal of contaminants, including bacteria and other harmful water particles.
Rico aims to further develop her research with a view to sustainability and notes that the team’s work is far from over.
“The product is still a long way from being ready for market,” she said. “While the rubbery material is cheap and easy to obtain, the oily phase is quite expensive. A less expensive pair of materials would be required here.” Perhaps DeepMind’s deep learning tool could be of service.
The full study is published in the journal Nature Materials.
Get the TNW newsletter
Get the most important tech news in your inbox each week.